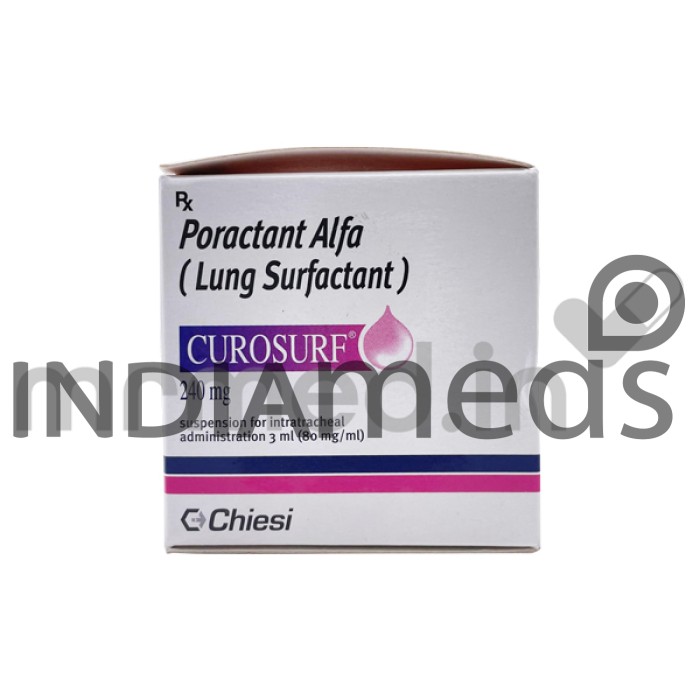
Curosurf 240mg injection contains active components such as Poractant alfa. It is a lung surfactant medication used to treat Respiratory Distress Syndrome (RDS) in premature infants. It is a lifesaving medication that has improved the survival and outcomes of many premature infants. It has been shown to reduce the risk of death, the need for mechanical ventilation, and the length of hospital stay in premature infants with RDS. It is a good option for infants who are at risk for developing bronchopulmonary dysplasia (BPD), a chronic lung disease that can develop in premature infants. It is more stable than other surfactant medications, which means it can be stored longer without losing effectiveness.
If someone is hypersensitive to Curosurf 240mg injection, they should not receive the medication. They may experience a severe allergic reaction, such as anaphylaxis. It should not be used for infants with severe RDS. This is because the medication may not be effective in these infants. This medication should not be given to infants with a history of porcine protein hypersensitivity. This medication can increase the risk of bleeding. Therefore, it should not be given to infants with active bleeding. It may not be effective in infants with neonatal pneumonia and meconium aspiration syndrome. Curosurf 240mg injection is not used in old age because it is ineffective in adults.
- Respiratory distress syndrome (RDS)
Therapeutic Effects of Curosurf 240mg Injection
Pregnancy
If it is always better to consult a doctor before using Curosurf 240mg injection if you are pregnant or planning to become pregnant.
Breast Feeding
Curosurf 240mg injection's safety during breastfeeding is not well established. Consult a healthcare provider before using this medication while breastfeeding.
Lungs
If you have lung conditions, it is advisable to consult your healthcare provider before Curosurf 240mg injection
Liver
Individuals with liver disease should consult a doctor regarding the use of Curosurf 240mg injection.
Alcohol
It is unsafe to take Curosurf 240mg injection with alcohol as it can increase the risk of bleeding and other complications.
Driving
Driving is unsafe after receiving Curosurf 240mg injection as it can cause drowsiness and dizziness.
Serious:
- Anaphylaxis
- Bleeding
- Pulmonary hemorrhage
- Intracranial hemorrhage
- Surfactant protein B deficiency
Common
- Bradycardia
- Hypotension
- Hypoxemia (decreased oxygen)
- Respiratory distress
- Apnea
- Froth at the mouth
- Irritation at the injection site
The effects of Curosurf 240mg injection typically last for 3 to 5 days. However, some infants may need to receive additional doses.
Curosurf 240mg injection is generally considered safe for premature infants. However, it is important to note that it can cause some side effects, such as bradycardia, hypotension, and hypoxemia.
If your infant needs more than one dose of Curosurf 240mg injection, they may be at an increased risk of side effects. However, the benefits of receiving Curosurf 240mg injection often outweigh the risks.
The long-term risks of Curosurf 240mg injection are not fully known. However, there is no evidence that it causes any long-term problems.
The prognosis for infants with RDS has improved significantly in recent years. With proper treatment, most infants with RDS make a full recovery.
Curosurf 240mg injection is similar to other synthetic surfactants in terms of its effectiveness in treating RDS. However, it is known to cause less bradycardia and hypotension than other synthetic surfactants.
Molecule name: Poractant alfa | Therapeutic class: Pulmonary surfactants |
Pharmacological class: Surfactants | Indications: 1. Respiratory distress syndrome (RDS) 2. Prevention of RDS |