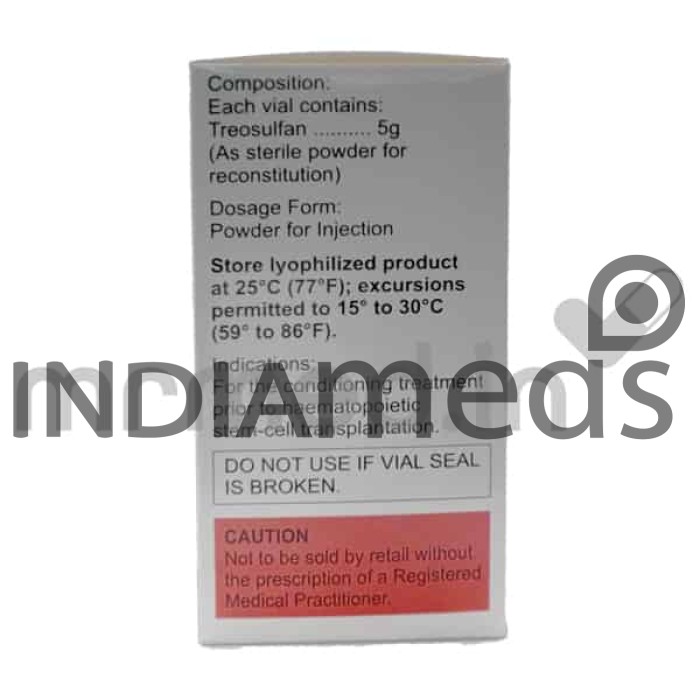
Emtreo 5gm injection contains the active substance Treosulfan. It is an anti-neoplastic medication belonging to the group called alkylating agents. This medicine is combined with Fludarabine to treat allogeneic hemopoietic stem cell transplant (alloHSCT) in adults with both malignant and non-malignant. In pediatric patients (older than one month), it treats malignant.
Do not take Emtreo 5gm injection if you are allergic to Treosulfan or its other ingredients. Notify your doctor if you have any infections or disorders with the heart, lungs, liver, or kidney. Before starting the treatment, inform your doctor if you took any live vaccine or have Fanconi anemia (inherited bone marrow failure syndromes) or other DNA breakage repair disorders. This medicine may reduce the blood count (myelosuppression); your doctor will monitor your blood counts and liver enzymes regularly to detect their functions until recovery of the hematopoietic system.
Before taking a Emtreo 5gm injection, notify your doctor if you are pregnant or planning to get pregnant or breastfeeding. Use effective contraception during and after the last dose for six months of the treatment. Tresosulfan impacts fertility impairment if advised not to father a child during and up to 6 months after treatment. In pediatric patients, dermatitis diaper (inflammation of the skin in the diaper area) may occur, so napkins should be changed frequently for 6–8 hours. Contact your doctor for more information.
Therapeutic Effects of Emtreo 5gm Injection
Pregnancy
Emtreo 5gm is unsafe to use during pregnancy as it may harm the unborn baby. Report to your doctor if you are pregnant or think you may be pregnant or planning for the pregnancy before starting the treatment.
Breast Feeding
Breastfeeding is not recommended in Emtreo 5gm patients because it is unknown whether the medicine passes into the breast milk. So, do not breastfeed during the treatment.
Lungs
Inform your physician about any underlying lung disorders before taking Emtreo 5gm, as this medication should be used cautiously in lung disease patients. Consult your physician before taking this medicine.
Liver
Inform your physician about any underlying liver disorders before taking Emtreo 5gm, as this medication should be used cautiously in liver disease patients. Consult your physician before taking this medicine.
Alcohol
It is unknown whether consuming alcohol while taking a Emtreo 5gm tablet is safe. Please speak with your physician.
Driving
While taking Emtreo 5gm, driving or using machines is unsafe because it may cause nausea, changes in vision, vomiting, etc. Talk to your doctor for more information.
Serious
- Decrease in blood cell count
- Infections
- Liver vien blockage
- Lung inflammation
Common
- Allergic reaction
- Decreased appetite
- Headache, dizziness
- Irregular heartbeat
- Breathing difficulties Joint pain
- Increased liver enzymes
- The lower level of bilirubin
Emtreo 5gm dosage is based on several factors, including the type and stage of the cancer being treated, the individual's overall health, and their response to the treatment.
One of the common side effects of Emtreo 5gm is the lower level of bilirubin. If you experience any side, contact your physician immediately.
The reason for this elevation is not completely understood, but it is thought to be related to the drug's effects on certain enzymes involved in liver function. The elevation in liver enzymes is generally mild and temporary and typically resolves on its own or with dose adjustment of the Emtreo 5gm tablet.
Do not miss your dose. Keep all your appointments with the dosing regularly. If you forget or miss an appointment, inform your healthcare professional and reschedule the dosing.
Even if you feel better, continue taking doxycycline until the prescription is completed. If you stop using Emtreo 5gm injections too soon or skip doses, your illness may not be entirely treated.
Molecule name: Treosulfan | Therapeutic class: Antineoplastics |
Pharmacological class: Alkalyting agent | Indications: Allogeneic hemopoietic stem cell transplant (alloHSCT) |