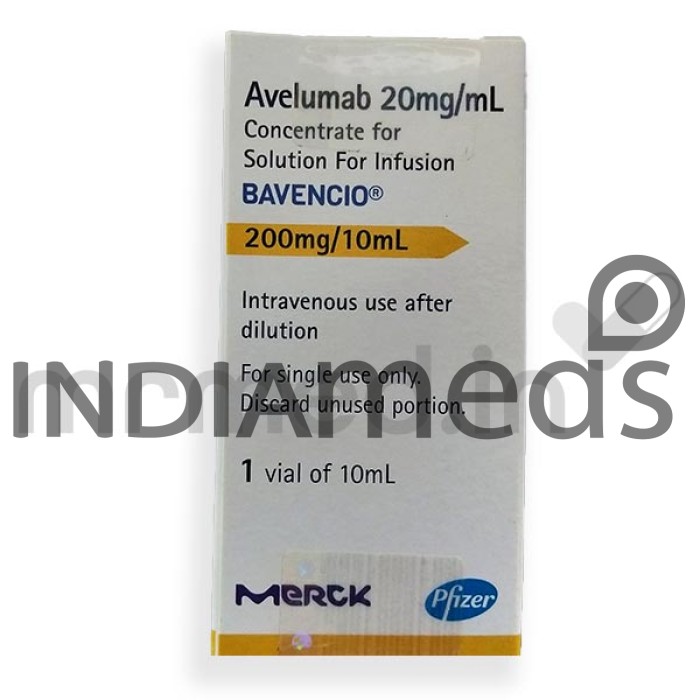
Bavencio 200mg/10ml injection contains the active ingredient avelumab. It is an antineoplastic drug that belongs to the class of medications called PD-1/PD-L1 (programmed death ligand-1) inhibitors. It is used to treat Merkel cell carcinoma, a rare type of skin cancer that has spread to other body parts in adults and children 12 years and older. It is also indicated to treat urothelial carcinoma, cancer that originates in the urinary tract that has spread to nearby tissues or other body parts of the body in people whose cancer worsened during or within 12 months after it was treated with other anticancer medications.
It is also used in combination with other anticancer medicines to treat renal cell carcinoma, a type of kidney cancer that has spread to other body parts. PD-L1 is found on the surface of certain cancer cells and helps protect them from the immune system. It works by slowing or stopping the growth and spread of the cancer cells. Do not take Bavencio 200mg/10ml injection if you are allergic to avelumab or any other ingredients of this medicine. Inform your physician if you have liver diseases, hepatitis B, HIV/AIDS, or other immune diseases before initiating the therapy.
This medicine is not recommended for children and adolescents below 18. If you suffer from any of the reactions like infusion-related reactions, inflammation of your lungs, liver, pancreas, watery stools, or diarrhea, inform your doctor immediately and seek medical attention. Bavencio 200mg/10ml injection should not be used in pregnant and breastfeeding women. Inform your physician if you are pregnant or breastfeeding. Use effective contraception throughout the therapy to avoid pregnancy. The common adverse reactions are tiredness, muscle pain, diarrhea, nausea, rash, decreased appetite, urinary tract infection, and swelling in the body parts.
Therapeutic Effects of Bavencio 200mg Injection 10ml
Pregnancy
Bavencio 200mg/10ml injection is unsafe to administer in pregnant women because it may affect the fetus. Inform your physician if you are pregnant, suspect pregnancy, or planning to become pregnant. Using an effective birth control method during the treatment and for 1 months after your last dose is necessary.
Breast Feeding
Breastfeeding is not recommended in patients taking Bavencio 200mg/10ml injection because the medicine passes into the breast milk in small amounts and may harm your baby.
Lungs
It is unknown whether Bavencio 200mg/10ml injection can be used in patients with lung disorders. Consult your doctor if you have any lung diseases before starting the treatment.
Liver
Bavencio 200mg/10ml injection is safe to administer in patients with liver problems. Dose adjustments are not necessary. However, inform your physician if you have any liver diseases or problems before initiating the therapy.
Alcohol
It is unknown whether consuming alcohol is safe during the treatment with Bavencio 200mg/10ml injection. Inform your doctor if you are a chronic drinker.
Driving
Bavencio 200mg/10ml injection may make you feel tired, dizzy, or sleepy. It is unsafe to drive or operate heavy machinery after taking this medicine.
Common
- Increased blood pressure
- Swelling in the legs and feet
- Skin rash
- Abdominal pain
- Decrease in appetite
- Diarrhea
- Nausea
- Decreased RBC counts
- Muscle pain
- Cough, shortness of breath
- Difficulty in talking
- Tiredness
Serious
- Heart problems
- Severe skin reactions
- Thyroid problems
- Liver injury
- Visual impairment
- Infusion reactions
Bavencio 200mg/10ml injection is given as a cycle therapy. Your doctor will decide the dose and duration of the therapy based on your disease severity and condition.
Certain medications may interact with the effectiveness of Bavencio 200mg/10ml injection including prescription, over-the-counter medicines, vitamin or nutritional supplements, and herbal products. Inform your physician about your medication history.
Heart problems like cardiac arrest and congestive heart failure may occur in patients receiving this medicine for the long term. Inform your physician of any pre-existing heart disorders before initiating the treatment.
The FDA has a patient information leaflet containing a summary of the essential scientific information needed for the safe and effective use of the drug. Read the information leaflet thoroughly before starting the therapy.
Signs of shortness of breath, wheezing, chills or shaking, rash, flushing, low blood pressure, dizziness, fatigue, nausea, fever, back pain, and abdominal pain are the infusion reactions that may cause due to the Bavencio 200mg/10ml injection administration. Inform your physician and seek medical attention immediately if you face these reactions after administering the injection.
Molecule name: Avelumab | Therapeutic class: Antineoplastics |
Pharmacological class: PD-1, PD-L1 inhibitors | Indications: 1. Merkel cell carcinoma 2. Urothelial carcinoma 3. Renal cell carcinoma |





-200x200.jpg)